Non-Small Cell Lung Cancer NSCLC is the most common form of lung cancer and its management depends on how far the disease has spread, the histology of the tumor, and the presence of specific molecular features. In recent years the landscape of treatment has become more personalized, with options ranging from surgery to systemic therapies that target particular mutations or harness the immune system. Alongside advances in therapy, recognizing signs early can influence outcomes by enabling earlier evaluation and treatment planning.
Common signs and symptoms of NSCLC can include a persistent cough that lasts for weeks, chest pain or discomfort, shortness of breath, wheezing, coughing up blood, hoarseness, unexplained weight loss, and fatigue. Some individuals experience recurring chest infections or symptoms that seem to worsen with activity. It is important to note that early stage NSCLC may not cause noticeable symptoms and is sometimes found incidentally on imaging done for other reasons. If any of these signs appear and persist, a medical evaluation is recommended, especially in people with risk factors such as a history of smoking or exposure to occupational hazards.
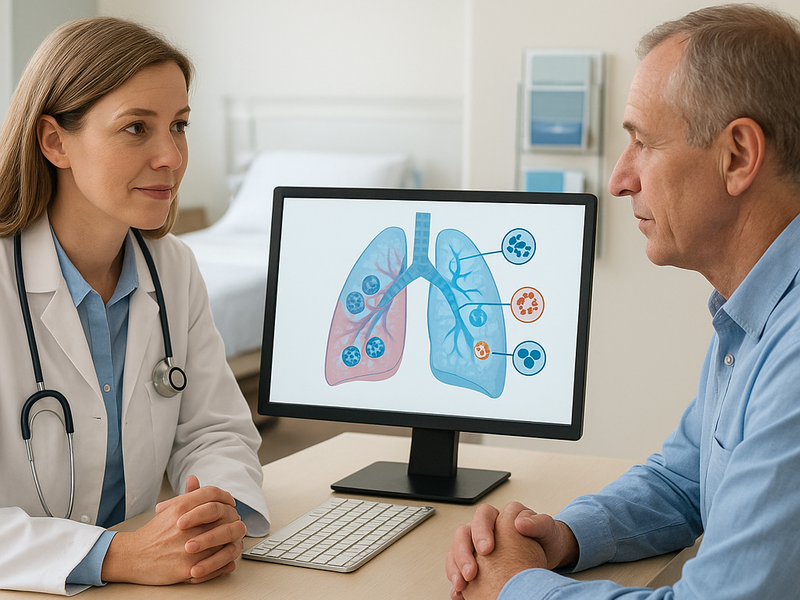
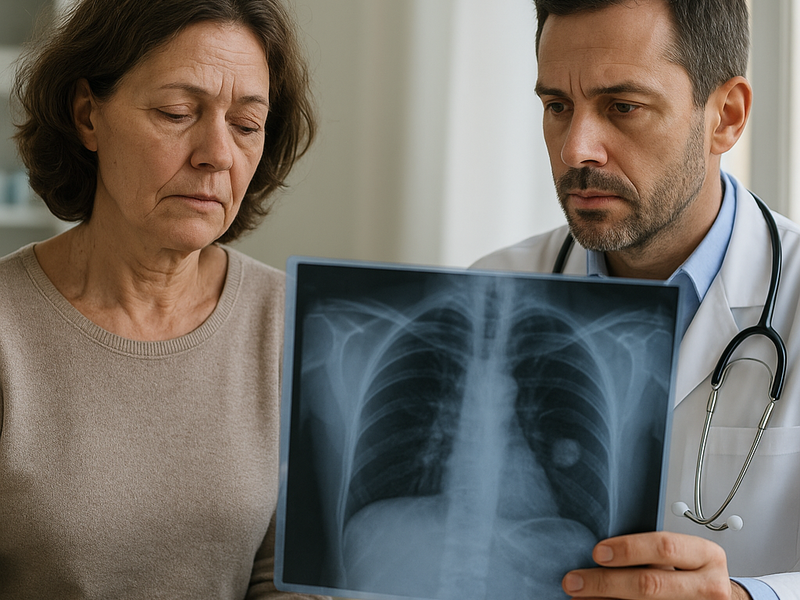
Diagnosis begins with imaging studies such as chest X rays and CT scans, followed by tissue sampling to determine histology and stage. A crucial part of the assessment is biomarker testing. Tests look for actionable mutations such as epidermal growth factor receptor EGFR mutations, ALK rearrangements, ROS1 fusions, BRAF alterations, and MET exon skipping, among others. PD-L1 expression is also evaluated in many cases because it can influence the use of certain immunotherapies. In some settings, liquid biopsy tests can detect tumor DNA in the blood to complement tissue results or monitor changes over time.
Treatment choices in NSCLC are increasingly tailored to the biology of the tumor and the stage at diagnosis. Surgery remains a cornerstone for many patients with early stage disease. Procedures such as lobectomy or limited resection aim to remove the tumor and nearby lymph nodes, when lung function and general health permit. Radiation therapy plays a pivotal role too, whether as a primary treatment for patients who cannot have surgery or as an adjunct to surgery or systemic therapy. Techniques like external beam radiotherapy and precision approaches such as stereotactic body radiotherapy allow high doses to targeted areas while sparing surrounding tissues.
Chemotherapy is still a central element of care for many patients, particularly when disease is more advanced or when combination regimens are chosen based on histology and molecular features. Platinum-based regimens, often paired with agents such as pemetrexed or paclitaxel, have been mainstays for decades and continue to be refined through clinical trials. The emergence of targeted therapies has transformed care for tumors with specific mutations. For example EGFR inhibitors including erlotinib, gefitinib, and osimertinib target cancers with EGFR mutations; ALK inhibitors such as crizotinib, ceritinib, alectinib, and brigatinib are used for ALK rearrangements; ROS1 fusions can be treated with crizotinib in many cases; BRAF alterations offer options with BRAF inhibitors in combination regimens.
Immunotherapy has become a major pillar for NSCLC, particularly for cancers that express high levels of PD-L1 or have other favorable features. PD-1 and PD-L1 inhibitors such as pembrolizumab, nivolumab, atezolizumab, and cemiplimab are used alone or with chemotherapy depending on stage, biomarker status, and patient factors. In locally advanced disease, durvalumab after chemoradiation is a common strategy to prolong control of the disease. While responses to immunotherapy can be durable for some patients, these treatments can cause immune related side effects that require careful monitoring and management.